An educator’s job is never done. Whether it’s the start or end of a school year, teachers and professors are constantly on the lookout for top-of-the-line microscopes for education. They want to equip their students with the tools they need to explore the unseen world around them, stimulate curiosity, and make incredible discoveries. Unfortunately, microscope shopping takes a great deal of time, and if they look in the wrong place, all their efforts could be for naught.
Fortunately, ACCU-SCOPE offers a wide selection of the best microscopes for educational environments. With ACCU-SCOPE, you will be able to outfit your classroom or school laboratory with the affordable and quality tools your students need — whether they’re high school students getting a first glimpse at biology or graduate school students conducting their own experiments.
The Top Microscopes for Middle School, High School, and College Students
As you shop for the classroom, you’ll recognize a variety of factors to consider before buying a student microscope. For example, you need to decide whether monocular or binocular microscopes are ideal for your students’ needs and which magnification levels will suit your application. In addition, you’ll need to consider the device’s frame, optics, lighting, field of view, and resolution. There’s a lot to think about!
ACCU-SCOPE created this easily digestible guide to help you buy the right microscopes for your classroom. Here are some of the best options for educational settings:
Middle school and high school students are still in the introductory phase to microscopes and science as a whole. This means they generally don’t require the advanced features or super high magnifications that may be more of a distraction than they are helpful. Of course, this doesn’t mean educators should compromise on quality. Great images and extreme durability are still important. That’s where the EXM-150 monocular microscope comes in.
The EXM-150 series provides great performance at an affordable price. Its student-proof design makes it the perfect instrument for any setting. Inexperienced students can make precise observations without having to fuss with various settings. The mechanical stage is highly secure, so you can depend on it for years to come. Even better, this microscope features LED lights that budding scientists can use with or without a cord, plus an integrated carrying handle, making the instrument easily transportable.
EXS-210 Stereo Microscopes
The EXS-210 series holds true to the adage, “Big things come in small packages.” This compact stereo microscope provides quality, versatility, and performance at an affordable price. It is student-proof, has corded or cordless operation, and is easily transportable, making classroom set-up a breeze. It also uses our state-of-the-art optical coating techniques and LED illuminators for high-quality images, durability, and dependability. This microscope is highly favored by middle and high school students, homeschoolers, and hobbyists.
If your classroom requires microscopes that can withstand years of rigorous use and you want binocular or trinocular viewing for greater comfort and image quality, you’ll want to consider the EXC-120 series. These microscopes are specifically engineered for clinical and veterinary applications, and classroom laboratories, making them ideal for college and graduate students. An EXC-120 microscope features high-contrast objectives, a die-cast frame, ergonomic focusing controls, brass gears, and a wide field of view. With this microscope, it’s never been easier to achieve clear images while observing your specimen in a comfortable position.
3079 Stereo Microscopes (Dissecting Microscopes)
If your students are ready to take their observations to the next level, the 3078 stereo microscope series is the best option for you. Its top-of-the-line optical system provides high resolution, 3-dimensional views of samples, while its ergonomic and durable design makes it ideal for long-term use. These microscopes are frequently found in university laboratories and industrial and OEM applications.
Going Digital: The Future of Microscopy in the Classroom
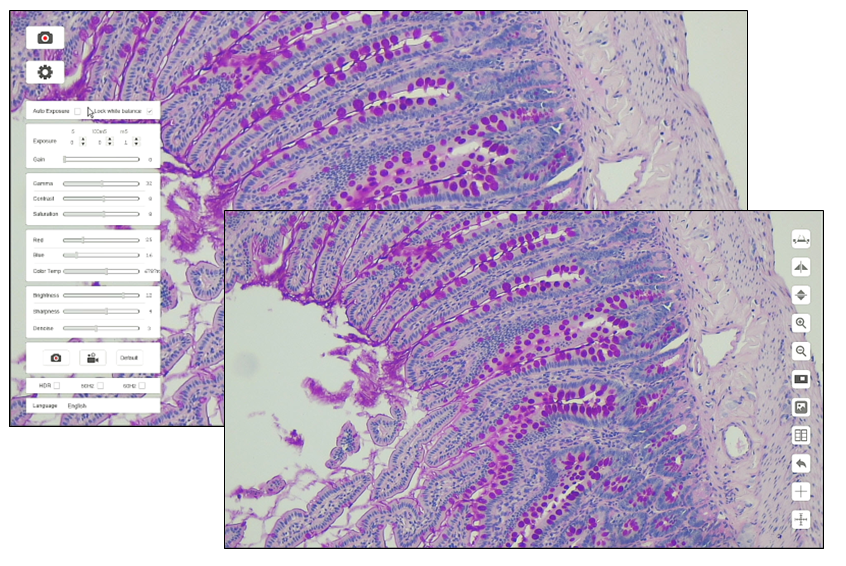
Whether you’re buying microscopes for high school or college labs, you will want to consider equipping the classroom or laboratory with digital microscopes. These microscopes either have a camera already installed or have connections that allow for the attachment of an LCD camera. With these modern instruments, you can demonstrate important scientific concepts in real time by projecting the image on a screen for all to see. Your students can also easily share their findings by saving the images or videos for a report.
We recommend the following digital cameras for educational purposes:
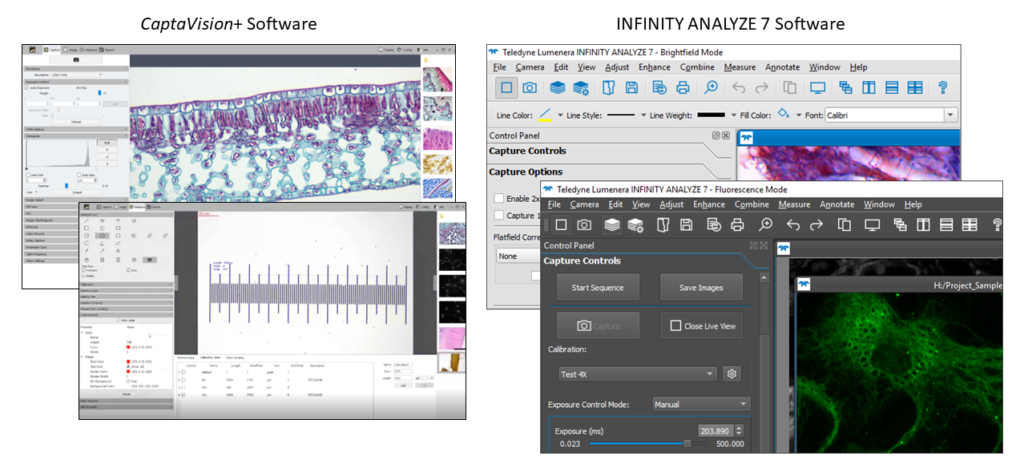
- Excelis HD Lite Camera: This camera works with most microscopes and allows HD live imaging of samples. The built-in software allows streamlined operation without a computer. The user can also capture images using CaptaVision+ software for editing and measuring at a later time.
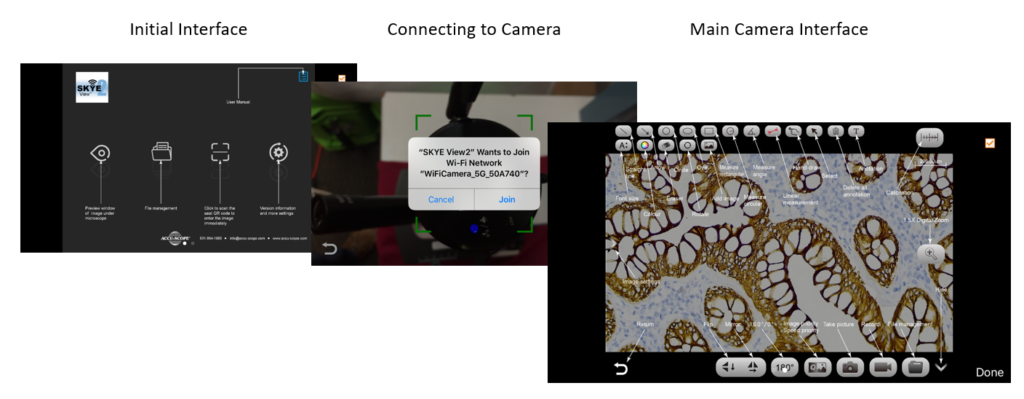
- SKYE WiFi 3 Camera: This camera creates its own WiFi network so the user can easily stream live images to a range of connected Android and iOS devices. Simply download the SKYE View 2 app, scan the QR code on the camera, and you’re ready to go! Thirteen students or more can connect to a single camera and view images simultaneously.
- Excelis 4K Camera: This camera delivers high-definition color images to any 4K monitor and the built-in software allows operation without a PC. Alternatively, the user can connect the camera to a PC using a USB 3.0 cable and control the camera through the CaptaVision+ imaging software.
Get the Best Microscope for Your Classroom Today
No matter your education needs, ACCU-SCOPE has a microscope that is perfect for you! We have an expansive range of educational microscopes plus the expertise to help you decide which ones will meet your students’ needs best. We continue to expand our product line to develop and include the latest advancements in the industry. Contact us today to learn more about our educational microscopy solutions and keep an eye out for new product introductions.